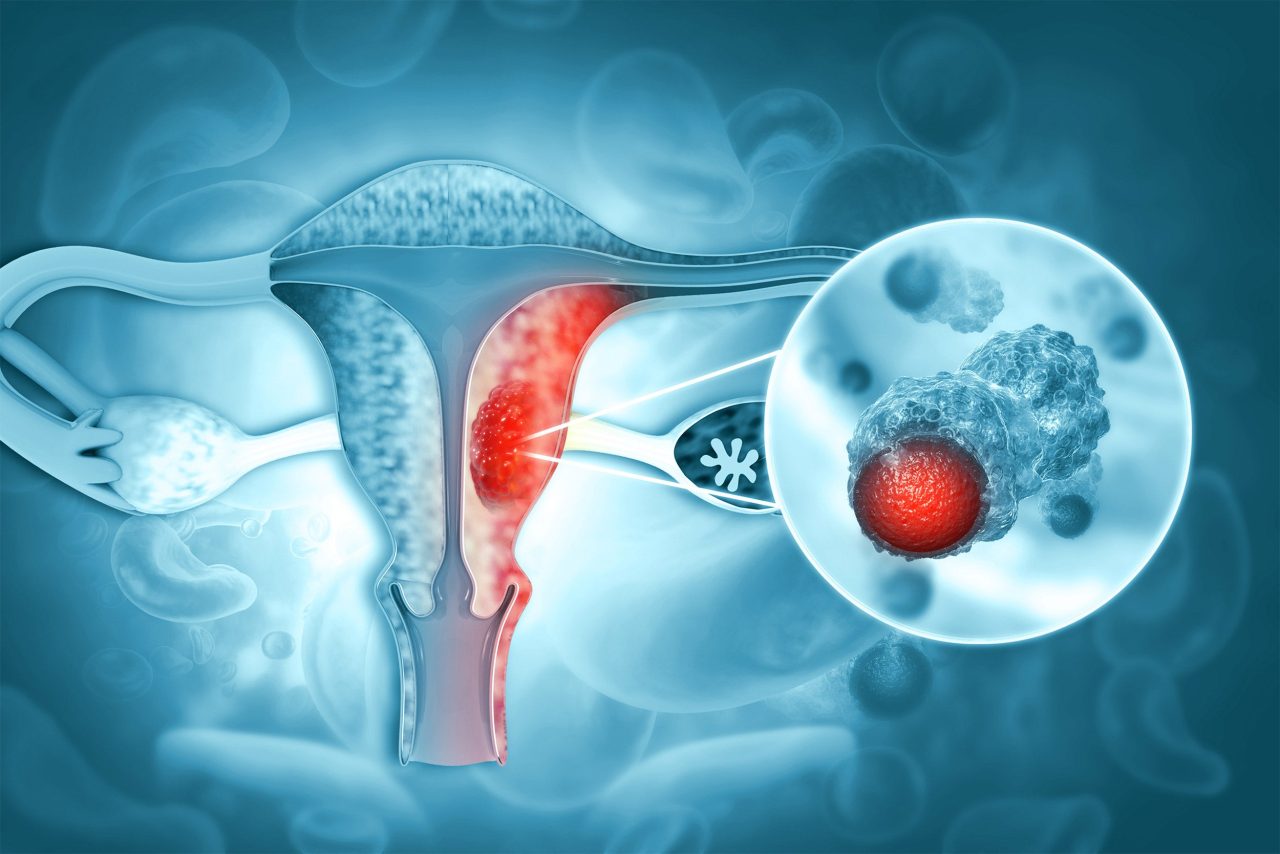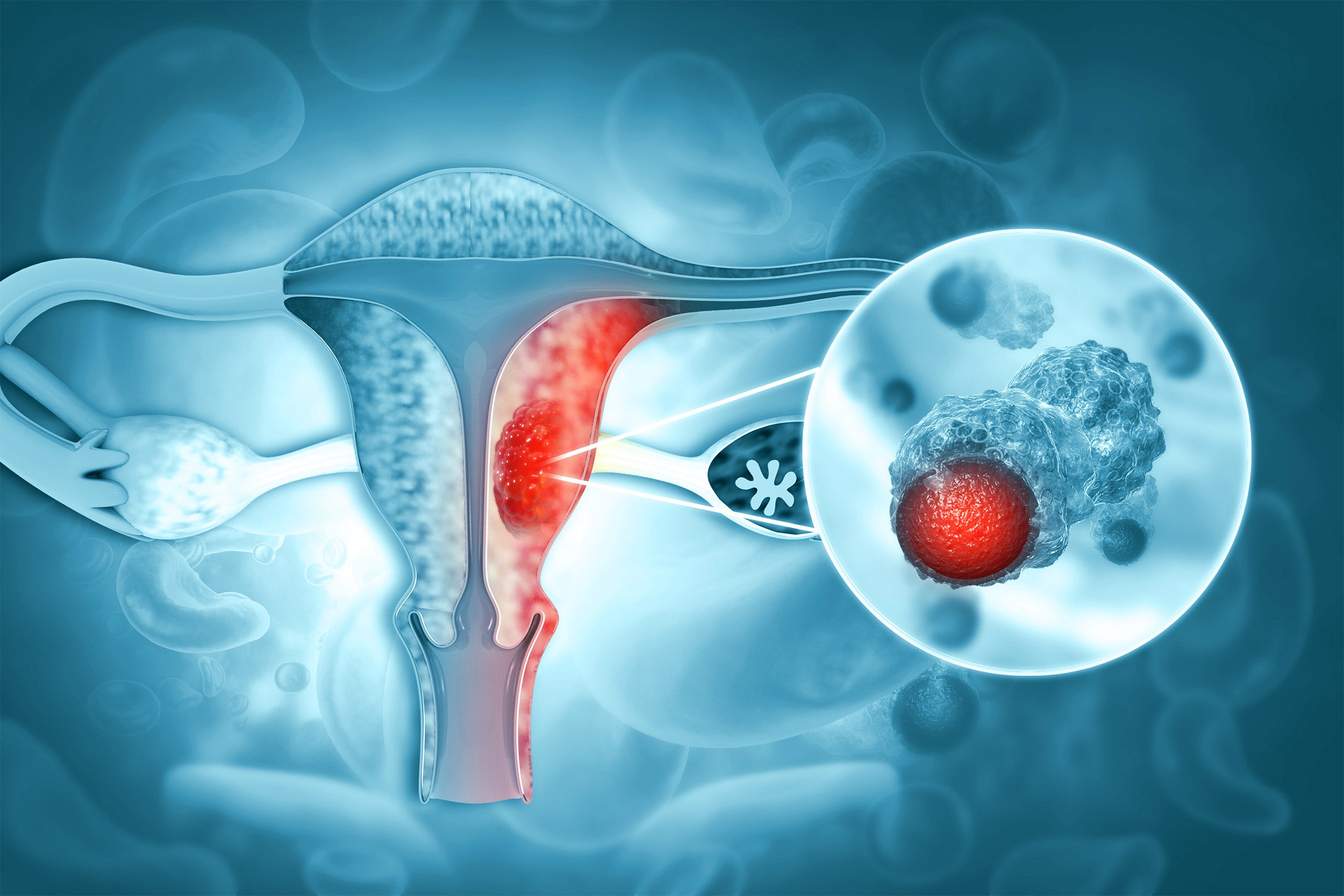
Endometrial malignant tumor.
iStock by Getty Images
Health
Model predicts risk of endometrial cancer
Current screening guidelines ignore those at high risk for the most prevalent gynecological malignancy in U.S.
A model to identify those at high risk for endometrial cancer has been developed by a Harvard-led team of investigators.
Despite endometrial cancer being the most common gynecological malignancy in the U.S., current guidelines do not recommend screening high-risk individuals, such as those with Lynch syndrome.
Using the largest, most heterogeneous international study population to date, investigators from Harvard-affiliated Brigham and Women’s Hospital, Harvard Medical School, and Harvard T.H. Chan School of Public Health, have developed a predictive model that can be translated into research and eventually clinical settings to identify high-risk individuals who would benefit from screenings.
“Given the rising incidence and mortality rate of endometrial cancer, population screening to identify high-risk women is an attractive public health strategy,” said first author Joy Shi of the Department of Epidemiology at Harvard School of Public Health. “I was interested in leveraging the incredibly rich questionnaire and genetic data available from the Epidemiology of Endometrial Cancer Consortium (E2C2) and combining it with other nationally representative data sources to assess whether we could improve on our ability to predict endometrial cancer risk in the general population.”
The model was trained on pooled data from an international consortium spanning 19 studies across different countries before being validated in three large U.S.-based cohorts. Their results are published in the Journal of the National Cancer Institute.
The only two previous risk models for endometrial cancer were trained on much more selective study populations and did not include risk factors like education, history of diabetes, history of hypertension, or use of hormonal therapy. To the author’s knowledge, this is also the first to assess the utility of genetic factors in endometrial cancer risk prediction.
“We found that epidemiologic risk factors alone … could accurately distinguish between women at high and low risk for endometrial cancer.”
Joy Shi, Harvard T.H. Chan School of Public Health
Shi and colleagues began by using data from 19 case-control studies involving postmenopausal white women between ages 45-85 in the E2C2 to develop their model.
They used a statistical tool called a LASSO model to predict which individuals would be at a higher risk of endometrial cancer relative to others in the cohort. Then, they translated these relative risks into an absolute risk prediction to determine the probability that an individual, given their characteristics, develops endometrial cancer in the next 10 years.
The model was validated using data from 121,700 female registered nurses aged 30-55 years in the Nurses’ Health Study, 116,430 female registered nurses aged 25-42 years from the Nurses’ Health Study II, and 78,232 women aged 55-74 years from PLCO (Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial). Participants were followed for 10 years in all three validation cohorts. Questionnaires, death records, and medical records were used to confirm diagnosis of endometrial cancer by pathologists and physicians.
“NHS, NHSII, and PLCO are cohorts with uniquely rich datasets, with data on tens of thousands of participants over decades of follow-up,” said senior author Immaculata De Vivo, a professor of Medicine at BWH and HMS. “There is also genetic data available for many of these study participants, which allowed us to investigate the potential contributions of genetic factors in predicting endometrial cancer risk.”
Researchers found that, on average, cumulative risk of endometrial cancer for women between ages 45-85 was 5.4 percent, though the range went from a risk of 1.4-1.8 percent for women in the lowest decile of risk to 13.7 to 15.01 percent for women in the highest decile. They noted the epidemiologic model demonstrated moderate accuracy with only slight improvements when genetic factors were included.
“We found that epidemiologic risk factors alone, which could be quickly, cheaply, and easily collected in clinical or public health settings, could accurately distinguish between women at high and low risk for endometrial cancer,” Shi said.
When the risk model was applied to a more recent and representative U.S. population of white women, the model identified 2.5 percent of women with more than 20 percent cumulative risk of endometrial cancer between ages 40-85. Those in the 97th percentile of risk had a predicted lifetime risk comparable to that in Lynch patients, who are recommended annual screening.
These findings suggest that clinical guidance about endometrial cancer screening could be updated. However, since 95 percent of the training cohort and 93-95 percent of the validation cohorts consisted of white women, the model does not generate and validate race-specific estimates for non-white women. The E2C2 consortium has been awarded a grant to understand disparities in endometrial cancer mortality across racial subgroups.
Models will need to continually be recalibrated in new geographic, temporal, or population settings to account for rarer subtypes of endometrial cancer and make predictions in more diverse populations.
J. Shi is supported by a UCB Fellowship. L. Cook held a Canada Research Chair and received career award funding from AHFMR. C. Friedenreich received career awards from the Canadian Institutes of Health Research and the Alberta Heritage Foundation for Medical Research (AHFMR) during the conduct of this study. A.B. Spurdle and P.M. Webb are supported by NHMRC Investigator Grants (APP177524; APP1173346). The E2C2 Data Coordinating Center at Memorial Sloan Kettering Cancer Center and multiple authors are supported by the National Cancer Institute grant U01 CA250476. The Data Coordinating Center is additionally supported by NCI P30 CA008748. I. De Vivo is supported by the Brigham Research Institute through the Fund to Sustain Research Excellence and supported by the Harvard Radcliffe Institute as their science faculty co-director. Funding sources for each individual study included in this analysis are provided below.